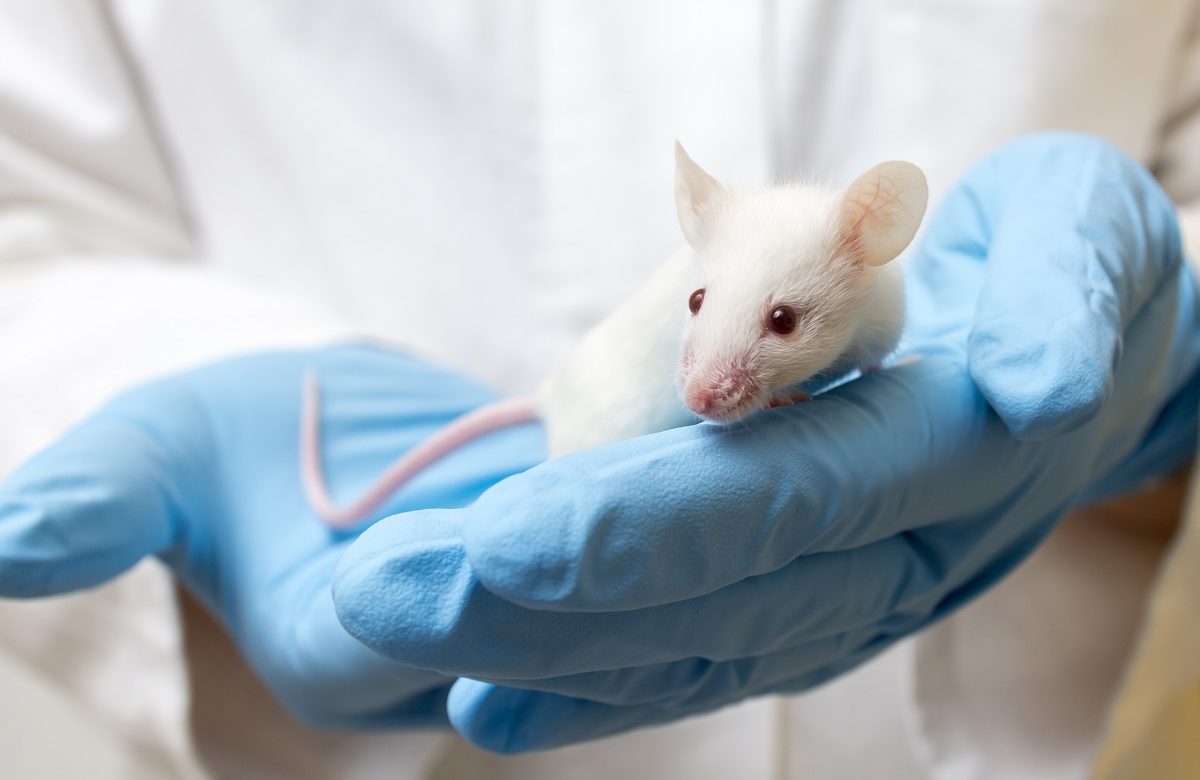
The National Experimental Biology Laboratory (NEBL) provides animal care and housing services to the university community and private companies. Its qualified personnel (preclinical scientists, veterinarians, and veterinary technicians) also offer preclinical research expertise on a multitude of animal species, including the development of new vaccines and drugs against emerging or progressive diseases.
The NEBL is the only laboratory at a Canadian university to be accredited by both the Canadian Council on Animal Care (CCAC) and the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC).
Facility description
The NEBL is one of only a handful of animal facilities in Quebec. It is located on the campus of the Armand-Frappier Santé Biotechnologie Research Centre of the Institut national de la recherche scientifique (INRS).
Located within the Biotech City in Laval, this state-of-the-art facility is accredited for biomedical and preclinical research. The research conducted at the NEBL is subject to rigorous supervision and is carried out in full compliance with international standards on the protection and living conditions of animals, as well as environmental protection.
The NEBL offers housing services for various species of animals at biosafety levels 1 and 2. It offers its clients a research environment under secure, controlled, and confidential conditions.
REQUEST A COTE
Animal housing
- 24/7 secure access
- 74 animal housing rooms
- Regular and specialized housing of animals ranging from rodents to non-human primates (including quarantine if necessary)
- Housing and living expenses (per day) including basic care, food and water, cage cleaning, clinical monitoring, and personal protective equipment
- One-off and annual rental of housing rooms (per day), including basic care, cage cleaning, and clinical monitoring
Equipment
Available for rent:- On-site laboratories and equipment (CL1 and CL2)
- Surgical suite and two necropsy rooms (daily rate)
- Cages for rodents, rabbits, dogs, or primates for external customers
Services
- Animal experiments (goats, dogs, guinea pigs, turkeys, ferrets, frogs, hamsters, rabbits, sheep, shrews, pigs, chickens, chicks, rats, monkeys, mice)
- Animal handling training available to users
- Training on rodent colony management
- Animal purchasing service (permits and logistics)
- Support for preclinical studies
- Technical assistance for the development of animal models other than rodents
- HPV decontamination
- Decontamination and sterilization (high capacity)
- Surgical suite rental (daily rate)
- Short- and long-term cage rental (monkeys, ferrets, guinea pigs, rabbits)
- Cage cleaning (high capacity)
- Colony maintenance and management for various animals
- Veterinary quarantine service for all animal species
- On-site veterinarians
- Technical support for the development of large-animal, small-animal, and livestock models
- Animal species
- Large animals: pigs, mini-pigs, dogs, sheep, ferrets, guinea pigs, hens, chicks
- Rodents: mice (including transgenic, nude, and SCID), rats (including transgenic, nude, and SCID), hamsters
- Zebrafish
- Non-human primates: cynomolgus macaques, rhesus macaques, capuchin monkeys, squirrel monkeys
- Other species: goats, turkeys, ferrets, rabbits, fish, chickens, chicks, cotton rats
- Assorted statistical analyses
- Wound healing models
- In-vivo pharmacokinetics
- Dietary exposure and intake assessments
- Bacterial infection models
- Viral infection models (e.g., influenza)
- Vaccines
- Immunology
- Antibody production
- Streptozotocin diabetic model (rats or mice)
- Acute liver disease models
- Infectious disease models (bacterial and viral)
- Acute LPS response models
- Feed supplements and animal nutrition studies
- Food consumption patterns
- Milk and dairy nutrition
- Tissues and fluids of various species
- Specialized dosing routes (e.g., intravenous infusion, intranasal)
Why use animal models in research?
Biomedical research involves multiple steps before a drug or vaccine is brought to market. To evaluate the biological response induced by the drug or vaccine, experimental research very often requires the use of animal models. Given the multitude of physiological interactions involved, in vitro cellular approaches are not yet able to recreate an environment as complex as that of a human or animal body for the study of immune responses to a vaccine or drug.Animal protection
The INRS Institutional Animal Care Committee (CIPA) is composed of researchers, students, veterinarians, veterinary technicians, and members of the public who meet once a month. One of CIPA’s mandates is to ensure that animal experimentation complies with CCAC guidelines and that the staff and students performing the experimentation are adequately trained and follow the procedures and animal care best practices.Use of large animals
Large animals are used for research only when absolutely necessary. They represent only a tiny fraction of the animals used in research (less than 0.5% at INRS). Because they are intelligent and social, large animals are paired and we avoid housing them alone. Great apes (like chimpanzees and baboons) are not used in research at INRS.- Animal replacement in research (avoiding or replacing the use of animals)
- Reduction in the number of animals used
- Refinement of our methods to improve animal comfort
- Animal replacement in research (avoiding or replacing the use of animals)
- Reduction in the number of animals used
- Refinement of our methods to improve animal comfort
Contacts
National Experimental Biology Laboratory (NEBL)
Building #26
531 des Prairies Blvd.
Laval, Quebec H7V 1B7
Canada