- Research
Evolution of longitudinal division and multicellularity in oral bacteria.
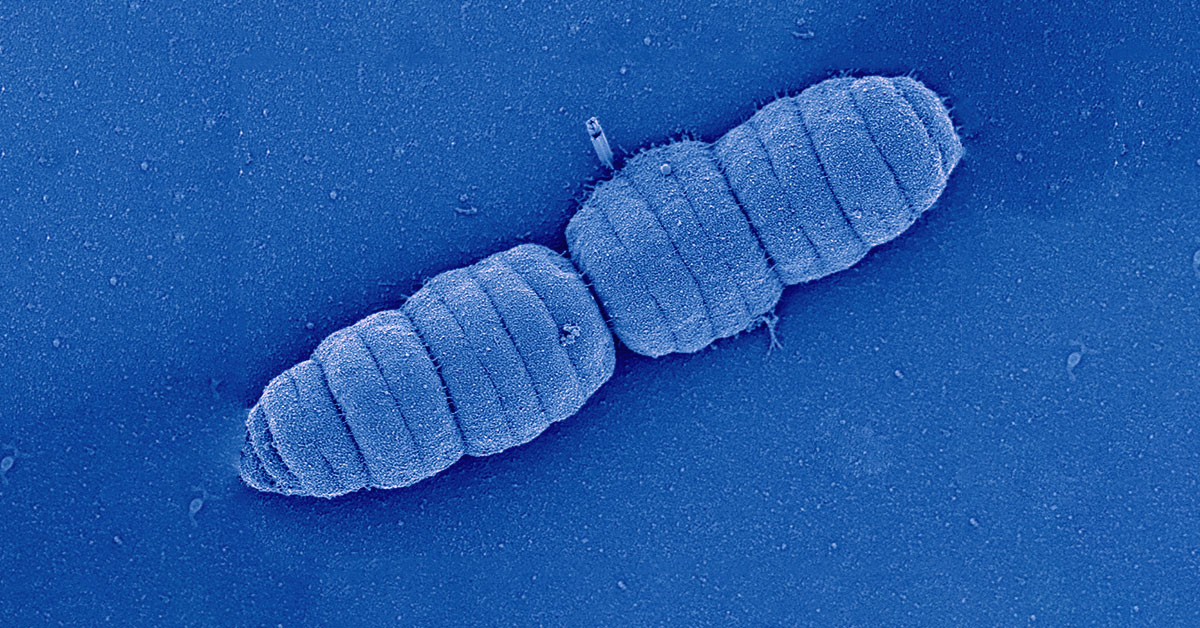
Scanning electron microscopy image of caterpillar-like bacterium Simonsiella muelleri (family Neisseriaceae) Credit: Sammy Nyongesa and Frédéric Veyrier (INRS).
Likely to survive in the oral cavity, bacteria evolved to divide along their longitudinal axis without parting from one another. A research team co-led by Professor Frédéric Veyrier, microbial geneticist from the Institut national de la recherche scientifique (INRS), and Professor Silvia Bulgheresi environmental cell biologist from the University of Vienna, just published their new insights in Nature Communications. In their work, they described the division mode of these caterpillar-like bacteria and their evolution from a rod-shaped ancestor. They propose to establish Neisseriaceae oral bacteria as new model organisms that could help pinpoint new antimicrobial targets.
Although our mouth houses over 700 species of bacteria and its microbiota is, therefore, as diverse as that of our gut, not much is known about how oral bacteria grow and divide. The mouth is a tough place to live in for bacteria. The epithelial cells lining the inner surface of the oral cavity are constantly shed and, together with salivary flow, organisms that inhabit this surface will therefore struggle for attachment. It is perhaps to better stick to our mouth that bacteria of the family Neisseriaceae have evolved a new way to multiply. Whereas typical rods split transversally and then detach from each other, some commensal Neisseriaceae that live in our mouths, however, attach to the substrate with their tips and divide longitudinally – along their long axis.
In addition to that, once cell division is completed, they remain attached to one another forming caterpillar-like filaments. Some cells in the resulting filament also adopt different shapes, possibly to perform specific functions to the benefit of the whole filament.
“Multicellularity makes cooperation between cells possible, for example in the form of division of labor, and may therefore help bacteria to survive nutritional stress.”
Frédéric Veyrier, INRS Professor who specializes in genomic bacteriology and evolution
Bacteria as new model organisms
The team of researchers first employed electron microscopy to survey bacterial cell shape across the Neisseriaceae family that include the two standard cell shapes (rod and coccus) in addition to the caterpillar-like filaments. By comparing their cell shapes and genomes throughout the Neisseriaceae family, they could infer that the multicellular, longitudinally dividing bacteria evolved out of rod-shaped, transversally dividing bacteria. Moreover, they could pinpoint which genes were likely responsible for the unusual multiplication strategy. They then used fluorescence labelling techniques to visualize the progression of cell growth in the multicellular bacteria and finally compared the genetic make-up of these with ‘classic’, rod-shaped species.
Finally, they tried to recreate that evolution by introducing the genetic changes into rod-shaped Neisseriaceae. Although they could not force rod-shaped bacteria to become multicellular, genetic manipulation resulted into longer and thinner cells.
“We speculate that in the course of evolution, through a reworking the elongation and division processes, the cell shape changed, perhaps to better thrive in the oral cavity”, says Prof. Veyrier.
“Apart from helping us to understand how cell shape evolved, multicellular Neisseriaceae may be useful to study how bacteria learned to live attached to the surface of animals, the only place they have been found to occur so far. Half of us is carrying them in our mouths, by the way”, explains Prof. Bulgheresi.
However, Philipp Weber from the University of Vienna, Bulgheresi’s PhD student who also worked on the study, highlights that “expanding the cell biology field to additional morphologies and symbiotic species is also crucial to increase the pool of protein targets (e.g., antibiotic targets) for biopharmaceutical applications.”
“An evolutionary approach, such as that undertaken here for the Neisseriaceae, can shed light on new, unforeseen protein targets.”
Sammy Nyongesa, INRS student and study’s first co-author (under the supervision of Prof. Veyrier)
About the publication
Nyongesa S.*1, Weber P.M.*1, Bernet È., Pulido F., Nieves C., Nieckarz M., Delaby M., Viehboeck T., Krause N., Rivera-Millot A., Nakamura A., Vischer N.O.E, vanNieuwenhze M., Brun Y.V., Cava F., Bulgheresi S.*2, Veyrier F.J.*2: Evolution of longitudinal division in multicellular bacteria of the Neisseriaceae family. *1authors contributed equally *2authors contributed equally In: Nature Communications, 2022. DOI: 10.1038/s41467-022-32260-w



