- Research
A discovery by an INRS doctoral student sheds light on the survival strategies of a formidable parasite

Ilona Gdovinova, doctoral student in virology and immunology at INRS and Professor Albert Descoteaux, director of the Infectiopole and lead author of the study. Photo: INRS
Leishmaniasis is a parasitic disease that affects up to 1 million people worldwide each year. It is caused by the protozoan Leishmania, transmitted through the bite of a sandfly. Once inside its host—human or animal—the parasite settles in immune cells called macrophages and multiplies within small bubble-like structures known as parasitophorous vacuoles.

To replicate, Leishmania needs lipids—essential molecules for many cellular functions. Contrary to previous beliefs, the amastigote form of the parasite, which multiplies in humans, does not produce its own lipids. Instead, it must extract them directly from the host cell.
This discovery, made by the team of Professor Albert Descoteaux at Institut national de la recherche scientifique (INRS), was recently published in PLoS Pathogens.
A Host Protein Hijacked for Parasitic Purposes
As part of her PhD research at INRS, Ilona Gdovinova uncovered a key mechanism by which Leishmania acquires a class of lipids known as sphingolipids. She demonstrated that the parasite hijacks a host macrophage protein called VAPA, which normally facilitates lipid transport between cellular compartments.
By observing infected macrophages, the team found that VAPA accumulates around the vacuoles containing the parasite. When this protein is blocked, the vacuole can no longer expand, and the parasite stops multiplying.
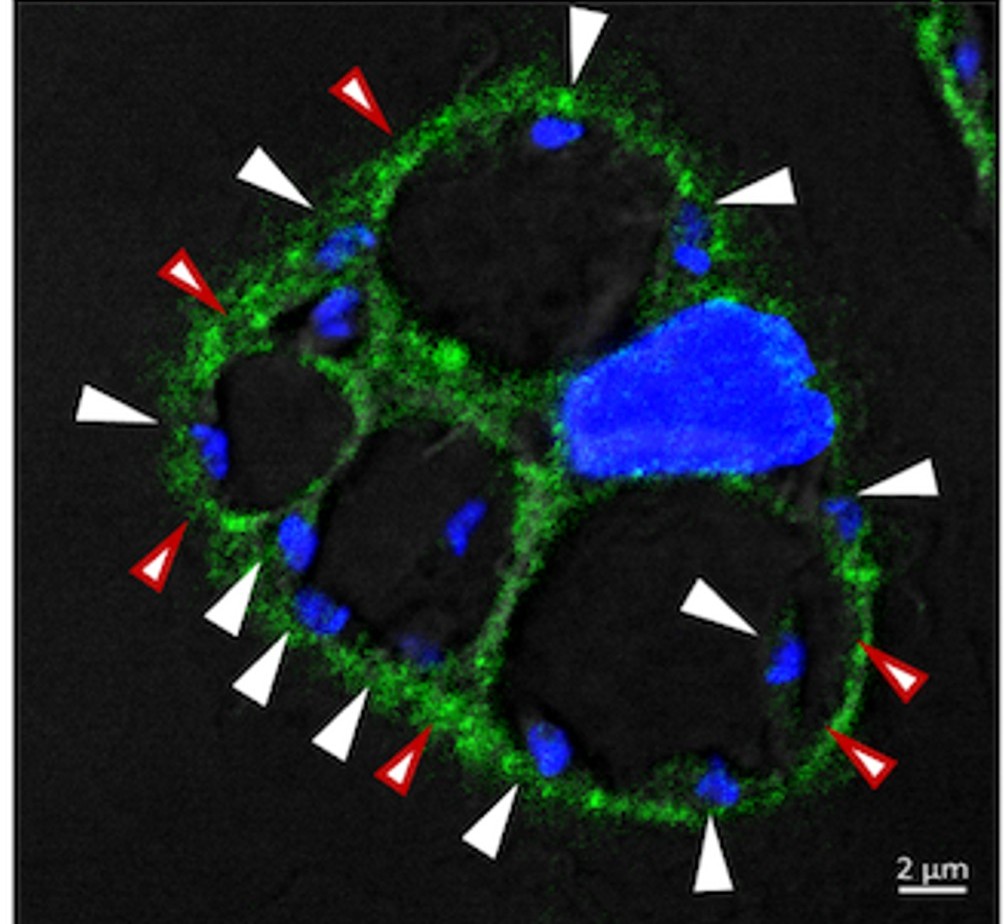
Macrophage infected with L. amazonensis. The VAPA protein (green) is present on the membrane of the L. amazonensis-harboring communal parasitophorous vacuole. White arrows indicate parasites, while red arrows highlight the recruitment of the VAPA protein to the parasitophorous vacuole.
Credit : Gdovinova, PLOS Pathogens 2025

“The parasite uses VAPA as a bridge to channel the lipids it needs. It even disrupts the protein’s normal interactions to better exploit it. This discovery highlights how finely parasites can manipulate cellular functions to ensure their survival”
Ilona Gdovinova, doctoral student in virology and immunology at INRS and first author of the study.
The team, based at the INRS Armand-Frappier Santé Biotechnologie Research Centre, also found that VAPA helps the parasite transport a key virulence molecule to another part of the host cell. This shows that the protein plays a crucial role in two-way exchanges between the parasite and its host.

“By identifying a critical mechanism for Leishmania’s acquisition of sphingolipids, this work opens the door to new strategies to block its replication”
Albert Descoteaux, professor at INRS, director of the Infectiopole and lead author of the study.
A specialist in host-pathogen interactions and a member of the Pasteur Network, Professor Descoteaux emphasizes the importance of understanding how these pathogens replicate in humans. This knowledge is essential for developing new therapeutic or preventive approaches—especially as drug resistance spreads and treatment options remain limited.
Leishmaniasis: Key Facts
- Endemic in South America, Southern Europe, Africa, and India
- Three main forms: visceral (most severe), cutaneous (most common), and mucocutaneous
- 700,000 to 1 million new cases annually, with 20,000 to 30,000 deaths (source: WHO)
- Closely linked to poverty, malnutrition, population displacement, and limited access to healthcare
About the Study
Gdovinova I, Descoteaux A (2025). VAPA mediates lipid exchange between Leishmania amazonensis and host macrophages. PLoS Pathog 21(3): e1012636. https://doi.org/10.1371/journal.ppat.1012636
This research was supported by the Canadian Institutes of Health Research (CIHR).



